A team at the Pacific Northwest National Laboratory (PNNL) has developed an improved molten–salt scheme for energy storage. The team claims that its “freeze–thaw battery” is a step toward creating batteries suitable for seasonal storage.
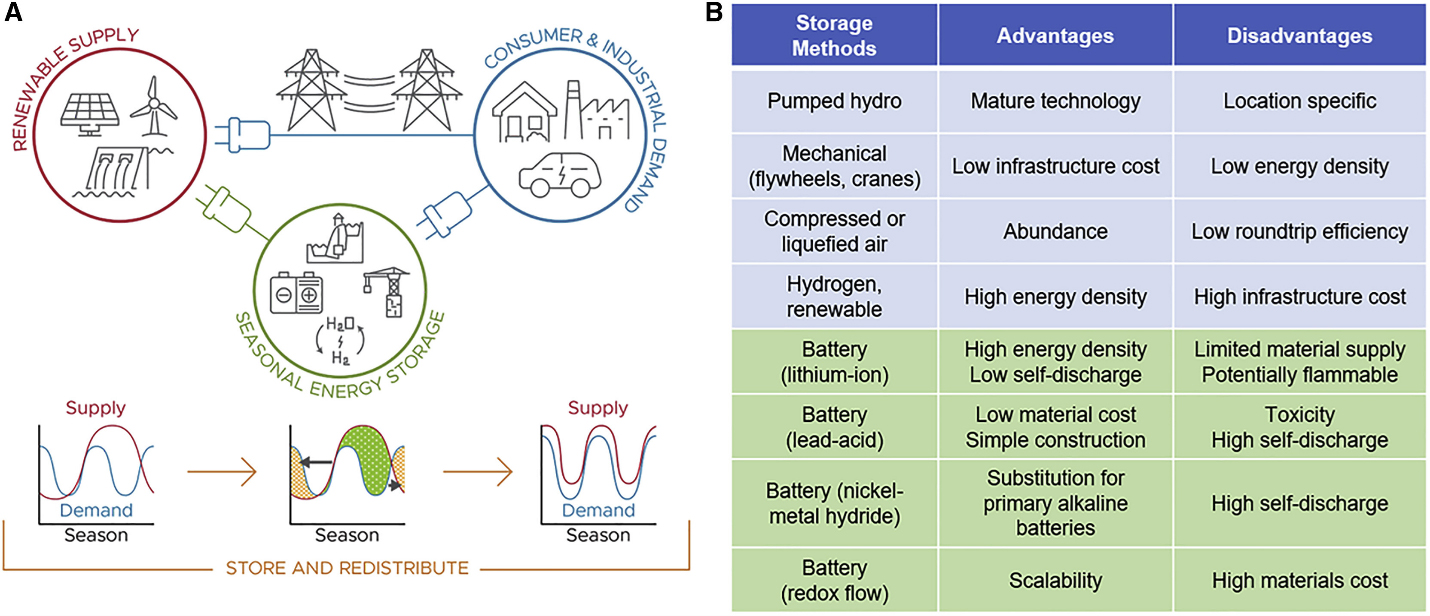
Any engineer involved in the path from energy acquisition to final use knows that there are three broad aspects to that path: energy capture/harvesting, storage, and transmission to the load. This is true regardless of the scale, whether a low–power intermittent load for a small IoT device or a large grid–scale arrangement. Depending on the specifics of the application and its size, the energy path will have these three elements in different proportions, with each having its own unique issues.
The storage part of the mix is extremely challenging, of course, especially in the context of renewable sources such as solar and wind power, where the source is intermittent while the user demands are not. In addition to cost and reliability, an important attribute of a viable storage scheme is that it has reasonably high energy–storage density by volume and weight. But this brings risks as well.
The quest for better ways to store energy is being pursued along many avenues: electrochemical (batteries), gravity (water and weights), and dynamic mechanical (flywheels) approaches as shown in Figure 1, to cite a few.
The PNNL team’s research, funded by Imre Gyuk, director of Energy Storage at the Department of Energy’s Office of Electricity, has resulted in an improved molten–salt scheme for energy storage. This is not the first use of molten slats for this purpose, however, as both the idea and various implementations have been known for decades.
The authors maintain that what’s different here is that their “freeze–thaw battery” is a step toward batteries that can be easily used for seasonal storage: saving energy in one season, such as spring, and using it in another, such as fall. The battery is first charged by heating it to 180⁰C, which allows ions to flow through the liquid electrolyte to create stored chemical energy.
Then, the battery is cooled to room temperature, which has the effect of “locking in” the battery’s energy. The electrolyte becomes solid and the ions that shuttle energy stay nearly still. The material is liquid at higher temperatures but solid at room temperature. When the energy must be accessed, the battery is reheated – presumably by natural seasonal warming – and the stored energy then becomes available.
I won’t go into the details of the salt material or electrochemical process, as they are fully presented in the PNNL team’s paper, “A freeze–thaw molten salt battery for seasonal storage”, published in Science Direct (plus – shhh! – chemistry is not one of my strong points). Their project investigated three somewhat–related activation methods of the nickel cathode in their battery for comparative purpose – an interesting perspective.
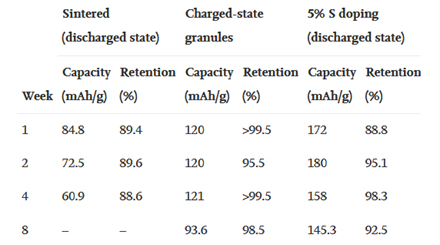
They provide some top–tier numbers based on their hockey puck–sized demonstration unit, as exampled in Figure 2. These storage blocks passively store the energy without much loss because the lack of mobility at the ambient temperature removes the self–discharge pathways.
The researchers claim a respectable capacity recovery over 90% after a period of one to eight weeks, adding that “the cells could effectively retain energy with comparable or superior performance to contemporary room temperature Li–ion batteries, which have low self–discharge rates at 2%–5% per month.”
An important benefit for this design is that the battery assembly and electrolyte use widely available materials rather than rare earths. The anode and cathode are solid plates of aluminum and nickel, while the separator is fiberglass rather than a more costly ceramic construction susceptible to cracking during freeze/thaw cycles. Finally, the materials (especially the electrolyte) do not pose the various risks of conventional batteries.
Reading through their paper (I’ll admit that much of the chemistry is beyond me), I didn’t get a clear sense of the traditional battery–like energy storage numbers, such as energy density by volume and weight, open cell voltage, current ratings, and power (rate of energy flow). That may be due to a lack of understanding on my part, or perhaps other reasons.
What’s your sense of the viability of this type of energy storage scheme? Is the idea of seasonal freeze/thaw practical, or only so in very limited circumstances — if at all? Do you think it can be scaled up in size and capacity – often the biggest challenge in any energy storage concept — even if it has been demonstrated as viable in a very small–scale test?




